 Bredt's Rule Bredt's Rule
 The importance of maintaining a planar configuration of the trigonal double-bond carbon components must never be overlooked. For optimum pi-bonding to occur, the p-orbitals on these carbons must be parallel, and the resulting doubly-bonded planar configuration is more stable than a twisted alternative by over 60 kcal/mole. The importance of maintaining a planar configuration of the trigonal double-bond carbon components must never be overlooked. For optimum pi-bonding to occur, the p-orbitals on these carbons must be parallel, and the resulting doubly-bonded planar configuration is more stable than a twisted alternative by over 60 kcal/mole.
 This structural constraint is responsible for the existence of alkene stereoisomers when substitutuion patterns permit. It also prohibits certain elimination reactions of bicyclic alkyl halides, that might be favorable in simpler cases. This structural constraint is responsible for the existence of alkene stereoisomers when substitutuion patterns permit. It also prohibits certain elimination reactions of bicyclic alkyl halides, that might be favorable in simpler cases.
 For example, the bicyclooctyl 3º-chloride shown below appears to be similar to tert-butyl chloride, but it does not undergo elimination, even when treated with a strong base (e.g. KOH or KOC4H9). For example, the bicyclooctyl 3º-chloride shown below appears to be similar to tert-butyl chloride, but it does not undergo elimination, even when treated with a strong base (e.g. KOH or KOC4H9).
 There are six equivalent beta-hydrogens that might be attacked by base (two of these are colored blue as a reference), so an E2 reaction seems plausible. The problem with this elimination is that the resulting double bond would be constrained in a severely twisted (non-planar) configuration by the bridged structure of the carbon skeleton. There are six equivalent beta-hydrogens that might be attacked by base (two of these are colored blue as a reference), so an E2 reaction seems plausible. The problem with this elimination is that the resulting double bond would be constrained in a severely twisted (non-planar) configuration by the bridged structure of the carbon skeleton.
 The carbon atoms of this twisted double-bond are colored red and blue respectively, and a Newman projection looking down the twisted bond is drawn on the right. Because a pi-bond cannot be formed, the hypothetical alkene does not exist. The carbon atoms of this twisted double-bond are colored red and blue respectively, and a Newman projection looking down the twisted bond is drawn on the right. Because a pi-bond cannot be formed, the hypothetical alkene does not exist.
 Structural prohibitions such as this are often encountered in small bridged ring systems, and are referred to as Bredt's Rule. Structural prohibitions such as this are often encountered in small bridged ring systems, and are referred to as Bredt's Rule.

 Bredt's Rule should not be applied blindly to all bridged ring systems. If large rings are present their conformational flexibility may permit good overlap of the p-orbitals of a double bond at a bridgehead. Bredt's Rule should not be applied blindly to all bridged ring systems. If large rings are present their conformational flexibility may permit good overlap of the p-orbitals of a double bond at a bridgehead.
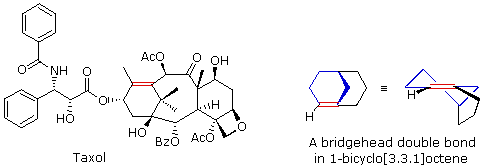
 This is similar to recognizing that trans-cycloalkenes cannot be prepared if the ring is small (3 to 7-membered), but can be isolated for larger ring systems. The anti-tumor agent taxol has such a bridgehead double bond (colored red), as shown in the following illustration. This is similar to recognizing that trans-cycloalkenes cannot be prepared if the ring is small (3 to 7-membered), but can be isolated for larger ring systems. The anti-tumor agent taxol has such a bridgehead double bond (colored red), as shown in the following illustration.
 The bicyclo[3.3.1]octane ring system is the smallest in which bridgehead double bonds have been observed. The drawing to the right of taxol shows this system. The bridgehead double bond (red) has a cis-orientation in the six-membered ring (colored blue), but a trans-orientation in the larger eight-membered ring. The bicyclo[3.3.1]octane ring system is the smallest in which bridgehead double bonds have been observed. The drawing to the right of taxol shows this system. The bridgehead double bond (red) has a cis-orientation in the six-membered ring (colored blue), but a trans-orientation in the larger eight-membered ring.
|